Both silicon and germanium can be used as the intrinsic semiconductor when fabricating solid-state devices. In the Periodic Table of the Elements, germanium (atomic number 32) occupies the position directly below silicon (atomic number 14).
The Periodic Table of the Elements had been envisioned earlier, but its potential was more fully realized in the work of Dmitri Mendeleev, an unusual individual to put it mildly. Born in a remote village in Siberia, he became renowned for his discoveries and writings including the comprehensive two-volume Principles of Chemistry (1868–1870).
He was a hard worker, highly educated, with many accomplishments in chemical engineering and related fields. Like many of his time and place, he was an unstable genius with a turbulent inner life. Fourteen years after marrying Feozva Nikitichna Lechcheria in 1862, he became obsessed over Anna Ivanova Popova and threatened suicide if she did not marry him. The stratagem succeeded but the event cast a long shadow across his life and was probably the reason he did not receive the Nobel Prize for his innovative Periodic Table.
The Periodic Table’s horizontal rows are known as periods and its vertical columns are called groups. The square cells each have the symbol (e.g. Fe for iron) signifying an element along with its atomic number, which equals the number of protons in its nucleus.
The period number is the highest energy level of an unexcited electron. By examining the position of an element within the Table one can ascertain the electron configuration including the number of shells and number of electrons in each shell, especially the number of electrons in the valence or outer shell. Various editions of the Table contain additional information such as atomic weight.
Cells are color coded, indicating the type of element, i.e. alkali metals, alkaline earth metals, transition metals, post-transition metals, metalloid, lanthanides, actinides, nonmetals, halogens and noble gases. Elements located in the same column (group) have identical valence populations. They are therefore chemically similar as this determines the ways in which they react with other elements.
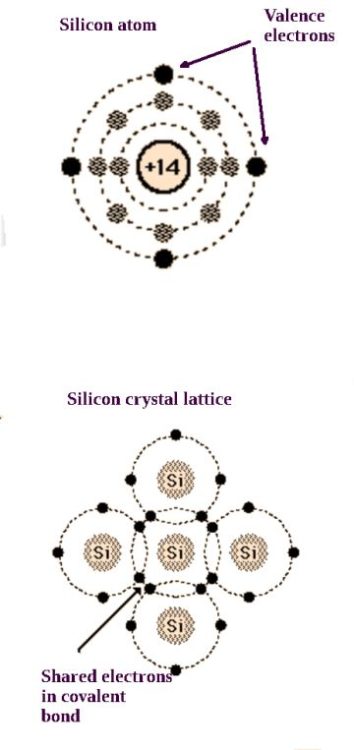
Since they are in the same column, we know that silicon and germanium have the same number of electrons in their outer or valence shell. Germanium atoms have one more shell than silicon atoms, but what makes for the interesting semiconductor properties is the fact that both have four electrons in the valence shell.
As a consequence, both materials readily constitute themselves as crystal lattices. Substituted atoms alter the electrical properties. The process of adding these atoms is known as doping. Doping may take place by passing a gas over the crystalline material, sometimes for several hours. If the dopant material is composed of atoms with five valence electrons, there will be extra free electrons and an n-type semiconductor is produced. If the dopant material is composed of atoms with three valence electrons, there is a deficiency of free electrons and a p-type semiconductor is produced.
Rather than saying there is a deficiency of free electrons, we can say the semiconductor has a surplus of holes. A hole is the absence of an electron. This may be simply a matter of semantics, but that is the customary terminology.
Dopants that have five valence electrons and make n-type semiconductors are antinomy, arsenic and phosphorous. Dopants that have three valence electrons and make p-type semiconductors are boron, aluminum and gallium. The same dopants are used for both silicon and germanium semiconductors.
Silicon is the principal component of common sand, and for this reason it is less expensive than other intrinsic semiconductor materials. But in such small quantities, raw material cost is not always decisive. Historically germanium was used as a semiconductor before silicon. The cat’s whisker RF detector could be found in early crystal sets. But in general, silicon is easier to process than germanium, able to handle higher power levels, has less reverse bias leakage and is more stable at higher temperatures.
Silicon and germanium can also be formed into an alloy of silicon-germanium with a molecular formula of the form Si1−xGex. Silicon-germanium serves as a semiconductor in integrated circuits for heterojunction bipolar transistors or as a strain-inducing layer for CMOS transistors.
Here heterojunction refers to the interface between two layers or regions of dissimilar crystalline semiconductors. The two semiconducting materials have unequal band gaps. (If their band gaps were equal, the interface would be a homojunction.)
SiGe lets CMOS logic integrate with heterojunction bipolar transistors. Heterojunction bipolar transistors have higher forward gain and lower reverse gain than typical homojunction bipolar transistors to help realize better low-current and high-frequency performance. Because it is a heterojunction technology with an adjustable band gap, SiGe enables more band-gap tuning than silicon-only technology.
SiGe-on-insulator (SGOI) is analogous to the Silicon-On-Insulator technology employed in computer chips. SGOI boosts the speed of transistors by straining the crystal lattice under the MOS transistor gate, improving electron mobility and raising drive currents. SiGe MOSFETs can also provide lower junction leakage because of the lower band-gap value of SiGe.

i want to know about the question why do we use si and ge as semicunductors not about thier position and other things related to thier position in periodic table ,so please answer the required question
That’s interesting to note that silicon can be found in sand. I guess that’s one of the reasons it’s so commonly used. I bet electronics makers try to use easily accessible materials often.
I didn’t know germanium was used as a semi-conductor before silicon. I guess we got really good at harvesting sand, since you mentioned silicon is the principal component of common sand. I bet factories use a lot of precisely made tools to make silicon chips. I know some chips get down to 0.05 mm sizes!
nice article
We use only si and ge because of their band gap properties, their ease of use and less cost when compared to other semiconductors like gallium arsenide, silicon arsenide etc… Hope u got it
First of all, David Herres, thanks for a great article! A very high-level treatise indeed on silicon and germanium in semiconductors. To address aditya’s complaint, I believe David answered your question very well. The explanation was so high-level, however, that I didn’t catch it all, and you may not have either.
jresquival’s point about using silicon because it’s cheap isn’t necessarily the case, as David mentioned that the amounts used are so small that the price of the element itself is not as important.
Want to thank Reshmasai for his astute observation and summation.
Thanks, everybody, for your comments! I learn a lot from all of them.
I want to know that the silicone and germanium both are semi-conducter but we only use the silicone in the solar system; why?